First Molar Eruption, Weaning, and Life History in Living Wild Chimpanzees
The Study in Brief
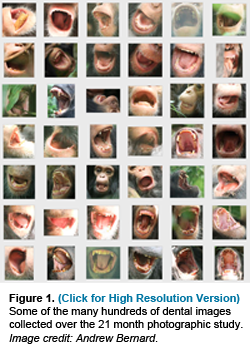
Scholars have been debating whether early humans (hominins) followed ape-like or human-like patterns of growth and development for quite some time. Others have recently questioned if developmental standards derived from captive animals are accelerated relative to wild animals, casting suspicion on most comparative data from great apes. Studies of human life history evolution often rely on broad primate-wide correlations of molar eruption age and developmental variables to assess fossil hominins, although these relationships have never been studied within the same population of primates. Using a novel photographic approach, a team of evolutionary anthropologists and wildlife photographers led by Professors Tanya Smith and Richard Wrangham, and Postdoctoral Fellow Zarin Machanda of Harvard's Department of Human Evolutionary Biology, have determined first molar (M1) eruption ages in several living chimpanzees. Our study demonstrates that healthy wild chimpanzees do not show systematic delays in M1 eruption age relative to captive chimpanzees, and both show strong similarities to early human ancestors. What is most surprising is that these ages do not correlate with weaning events or maternal reproductive states, but instead relate to the transition to an adult diet.